当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Using Tryptophan Mutants To Probe the Structural and Functional Status of BsSCO, a Copper Binding, Cytochrome c Oxidase Assembly Protein from Bacillus subtilis
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.biochem.7b00833 Shina Hussain 1 , Diann Andrews 1 , Bruce C. Hill 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.biochem.7b00833 Shina Hussain 1 , Diann Andrews 1 , Bruce C. Hill 1
Affiliation
|
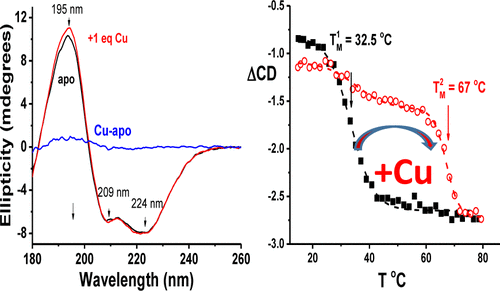
The synthesis of cytochrome c oxidase protein from Bacillus subtilis (i.e., BsSCO) binds copper with picomolar affinity, which increases the protein’s melting temperature (i.e., TM) by 20 °C. Here two native tryptophans (i.e., W36 and W101) are identified as major contributors to BsSCO’s structural form, and their contributions to the stability, intrinsic fluorescence, and copper binding properties of BsSCO are explored. Single mutations of tryptophan to phenylalanine decrease the TM by 10 °C and the folding free energy by 3–4 kcal/mol. A more severe change to alanine (i.e., W36A BsSCO) decreases the TM by 20 °C and the stability by 9 kcal/mol. However, these mutants bind copper with high affinity and assemble cytochrome c oxidase in vivo. Replacing phenylalanine at a position near (∼5 Å) the copper binding site with tryptophan (i.e., F42W) increases the TM of apo-BsSCO by 3 °C but diminishes the effect of copper binding. When both native tryptophans are changed to alanine, apo-BsSCO is unfolded in vitro and is not functional in cytochrome c oxidase assembly in vivo. A double-mutant of BsSCO in which W36A is combined with F42W exhibits a form of metastability. Apo-W36A/F42W BsSCO melts at 37 °C, which upon binding of copper shifts to 65 °C. B. subtilis expressing W36A/F42W BsSCO and grown at 37 °C does not assemble cytochrome c oxidase. However, when these cells are cooled to 25 °C, cytochrome c oxidase activity is recovered. Our results illustrate the subtle relationship between the structural stability and functional properties of BsSCO in the assembly of cytochrome c oxidase.
中文翻译:

使用色氨酸突变体来探测Bs SCO的结构和功能状态,Bs SCO是一种来自枯草芽孢杆菌的铜结合型细胞色素c氧化酶组装蛋白
由枯草芽孢杆菌(Bs SCO)合成细胞色素c氧化酶蛋白以皮摩尔亲和力结合铜,从而使蛋白的解链温度(即T M)增加20°C。这里有两个本地色氨酸(即,W36和W101)被确定为主要贡献者烧烤SCO的结构形式,以及它们与稳定做出了贡献,固有荧光,和铜结合的特性烧烤SCO进行了探索。色氨酸变为苯丙氨酸的单突变使T M降低10°C,折叠自由能降低3-4 kcal / mol。丙氨酸的更严重变化(即W36A Bs SCO)降低了20°C时的T M和9 kcal / mol的稳定性。然而,这些突变体以高亲和力结合铜并在体内组装细胞色素C氧化酶。在接近(〜5 a)用色氨酸的铜结合位点(即,F42W)的位置处替换苯丙氨酸增加Ť中号脱辅基的烧烤由3 SCO℃但减少铜的结合的影响。当两个天然色氨酸改变为丙氨酸,脱辅基烧烤SCO展开体外和不在细胞色素官能Ç氧化酶组装体内。的双突变烧烤W36A与F42W组合在一起的SCO表现出亚稳的形式。Apo-W36A / F42W Bs SCO在37°C时熔化,铜键结合后转变为65°C。表达W36A / F42W Bs SCO并在37°C下生长的枯草芽孢杆菌不能装配细胞色素C氧化酶。但是,当这些细胞冷却至25°C时,细胞色素c氧化酶活性得以恢复。我们的结果说明了Bs SCO在细胞色素c氧化酶组装过程中的结构稳定性和功能特性之间的微妙关系。
更新日期:2017-11-19
中文翻译:

使用色氨酸突变体来探测Bs SCO的结构和功能状态,Bs SCO是一种来自枯草芽孢杆菌的铜结合型细胞色素c氧化酶组装蛋白
由枯草芽孢杆菌(Bs SCO)合成细胞色素c氧化酶蛋白以皮摩尔亲和力结合铜,从而使蛋白的解链温度(即T M)增加20°C。这里有两个本地色氨酸(即,W36和W101)被确定为主要贡献者烧烤SCO的结构形式,以及它们与稳定做出了贡献,固有荧光,和铜结合的特性烧烤SCO进行了探索。色氨酸变为苯丙氨酸的单突变使T M降低10°C,折叠自由能降低3-4 kcal / mol。丙氨酸的更严重变化(即W36A Bs SCO)降低了20°C时的T M和9 kcal / mol的稳定性。然而,这些突变体以高亲和力结合铜并在体内组装细胞色素C氧化酶。在接近(〜5 a)用色氨酸的铜结合位点(即,F42W)的位置处替换苯丙氨酸增加Ť中号脱辅基的烧烤由3 SCO℃但减少铜的结合的影响。当两个天然色氨酸改变为丙氨酸,脱辅基烧烤SCO展开体外和不在细胞色素官能Ç氧化酶组装体内。的双突变烧烤W36A与F42W组合在一起的SCO表现出亚稳的形式。Apo-W36A / F42W Bs SCO在37°C时熔化,铜键结合后转变为65°C。表达W36A / F42W Bs SCO并在37°C下生长的枯草芽孢杆菌不能装配细胞色素C氧化酶。但是,当这些细胞冷却至25°C时,细胞色素c氧化酶活性得以恢复。我们的结果说明了Bs SCO在细胞色素c氧化酶组装过程中的结构稳定性和功能特性之间的微妙关系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号