Abstract
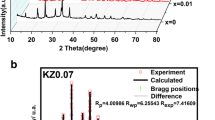
As the sodium host, the P2-NaxMnO2 cathode suffers from multiple structural transformations caused by the Jahn-Teller distortion, incurring rapid voltage decay and capacity degradation of the sodium-ion batteries. Herein, K and Ti elements have been co-doped into the P2-NaxMnO2 to adjust the Na+/vacancy ordering and to reduce the Jahn-Teller distortion-caused structural transformations by lowering the Mn3+ concentration. The prepared P2-Na0.66K0.01Mn0.9Ti0.1O2 shows high structural stability and rate performance with capacity retention of 87.4% after 200 cycles at a current density of 200 mA g−1, which is 60% higher than that of Na0.67MnO2. Moreover, the P2-Na0.66K0.01Mn0.9Ti0.1O2 also exhibits a good rate performance of 95.4 mAh g−1 at the same current density (74 mAh g−1 for Na0.67MnO2). The ex-situ XRD analyses demonstrate that the remarkable electrochemical performance of Na0.66K0.01Mn0.9Ti0.1O2 cathode should benefit from the high structural stability during the charge and discharge process and the improved diffusion kinetics.










Similar content being viewed by others
References
Zhu H, Yao Z, Zhu H et al (2022) Unblocking oxygen charge compensation for stabilized high-voltage structure in P2-Type sodium-ion cathode. Adv Sci n/a (n/a), 2200498
Kim H, Kim H, Ding Z et al (2016) Recent progress in electrode materials for sodium-ion batteries. Adv Energy Mater 6(19):1600943
Tarascon J-M (2010) Recent progress in electrode materials for sodium-ion batteries. Nat Chem 2(6):510–510
Steingart DA, Viswanathan V (2018) Is lithium the new gold? Energy Environ Sci 11(1):221–222
Yabuuchi N, Kubota K, Dahbi M et al (2014) Research development on sodium-ion batteries. Chem Rev 114(23):11636–11682
Zuo W, Qiu J, Liu X et al (2020) Highly-stable P2–Na0.67MnO2 electrode enabled by lattice tailoring and surface engineering. Energy Storage Mater 26:503–512
Liu X, Zuo W, Zheng B et al (2019) P2-Na0.67AlxMn1−xO2: cost-effective, stable and high-rate sodium electrodes by suppressing phase transitions and enhancing sodium cation mobility. Angew Chem-Int Edit 58(50):18086–18095
Saravanan K, Mason CW, Rudola A et al (2013) The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries. Adv Energy Mater 3(4):444–450
Yang Z, Li G, Sun J et al (2020) High performance cathode material based on Na3V2(PO4)2F3 and Na3V2(PO4)3 for sodium-ion batteries. Energy Storage Mater 25:724–730
Nakamoto K, Sakamoto R, Sawada Y et al (2019) Prussian blue-type electrodes: over 2 V aqueous sodium-ion battery with Prussian blue-type electrodes. Small Methods 3(4):1970010
Shan Y, Zhang G, Yin W et al (2022) Recent progress in Prussian blue/Prussian blue analogue-derived metallic compounds. Bull Chem Soc Jpn 95(2):230–260
Fang T, Guo S, Jiang K et al (2019) Revealing the critical role of titanium in layered manganese-based oxides toward advanced sodium-ion batteries via a combined experimental and theoretical study. Small Methods 3(4):1800183
Hemalatha K, Jayakumar M, Bera P et al (2015) Improved electrochemical performance of Na0.67MnO2 through Ni and Mg substitution. J Mater Chem A 3(42):20908–20912
Or T, Kaliyappan K, Bai Z et al (2020) High voltage stability and characterization of P2-Na0.66Mn1-yMgyO2 cathode for sodium-ion batteries. Chem Electro Chem 7(15):3284–3290
Zhang B, Zhang B, Wang L et al (2020) Li and Ti co-doping to stabilize slabs of high-voltage P2-type Na0.560[Li0.041Mn0.642Ni0.221Ti0.095]O2. J Alloy Compd 824:153938
Sehrawat D, Zhang J, Yu DYW et al (2019) In situ studies of Li/Cu-doped layered P2 NaxMnO2 electrodes for sodium-ion batteries. Small Methods 3(4):1800092
Guo S, Li Q, Liu P et al (2017) Environmentally stable interface of layered oxide cathodes for sodium-ion batteries. Nat Commun 8(1):135
Xia Y, Zhou Y, Yoshio M (1997) Capacity fading on cycling of 4 V Li / LiMn2O4 Cells. J Electrochem Soc 144(8):2593–2600
Lu J, Zhan C, Wu T et al (2014) Effectively suppressing dissolution of manganese from spinel lithium manganate via a nanoscale surface-doping approach. Nat Commun 5(1):5693
Quyen NQ, Van Nguyen T, Thang HH et al (2021) Carbon coated NaLi0.2Mn0.8O2 as a superb cathode material for sodium ion batteries. J Alloy Compd 866:158950
Billaud J, Singh G, Armstrong AR et al (2014) Na0.67Mn1−xMgxO2 (0 ≤ x ≤ 0.2): a high capacity cathode for sodium-ion batteries. Energy Environ Sci 7(4):1387–1391
Wu NHK, Peng Z et al (2020) Improved high rate performance and cycle performance of Al-Doped O3-Type NaNi0.5Mn0.5O2 cathode materials for sodium-ion batteries. J Phys Chem C 124(42):22925–22933
Tie D, Gao G, Xia F et al (2019) Modulating the interlayer spacing and Na+/vacancy disordering of P2-Na0.67MnO2 for fast diffusion and high-rate sodium storage. ACS Appl Mater Interfaces 11(7):6978–6985
Mao Q, Yu Y, Wang J et al (2021) Mitigating the P2–O2 transition and Na+/vacancy ordering in Na2/3Ni1/3Mn2/3O2 by anion/cation dual-doping for fast and stable Na+ insertion/extraction. J Mater Chem A 9(17):10803–10811
Wang C, Liu L, Zhao S et al (2021) Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium-ion battery. Nat Commun 12(1):2256
Zhao W, Tanaka A, Momosaki K et al (2015) Enhanced electrochemical performance of Ti substituted P2-Na2/3Ni1/4Mn3/4O2 cathode material for sodium ion batteries. Electrochim Acta 170:171–181
Shi Y, Li S, Gao A et al (2019) Probing the structural transition kinetics and charge compensation of the P2-Na0.78Al0.05Ni0.33Mn0.60O2 cathode for sodium ion batteries. ACS Appl Mater Interfaces 11(27):24122–24131
Wang P-F, Xiao Y, Piao N et al (2020) Both cationic and anionic redox chemistry in a P2-type sodium layered oxide. Nano Energy 69:104474
Cheng C, Hu H, Yuan C et al (2022) Precisely modulating the structural stability and redox potential of sodium layered cathodes through the synergetic effect of co-doping strategy. Energy Storage Mater 2022(52):10–18
Tosun SG, Uzun D, Yeşilot S (2021) Novel K+-doped Na0.6Mn0.35Fe0.35Co0.3O2 cathode materials for sodiumion batteries: synthesis, structures, and electrochemical properties. J Solid State Elec 25(4):1271–1281
Sehrawat D, Cheong S, Rawal A et al (2019) Investigation of K modified P2 Na0.7Mn0.8Mg0.2O2 as a cathode material for sodium-ion batteries. CrystEngComm 21(1):172–181
Qi X, Wang Y, Jiang L et al (2016) Sodium-deficient O3-Na0.9[Ni0.4MnxTi0.6−x]O2 layered-oxide cathode materials for sodium-ion batteries. Part Part Syst Charact 33(8):538–544
Zhang T, Ji H, Hou X et al (2022) Promoting the performances of P2-type sodium layered cathode by inducing Na site rearrangement. Nano Energy 100:107482
Rosseler O, Sleiman M, Montesinos VN et al (2013) Chemistry of NOx on TiO2 surfaces studied by ambient pressure XPS: products, effect of UV irradiation, water, and coadsorbed K+. J Phys Chem Lett 4(3):536–541
Feng J, Luo S-H, Wang J et al (2022) Stable electrochemical properties of magnesium-doped co-free layered P2-type Na0.67Ni0.33Mn0.67O2 cathode material for sodium ion batteries. ACS Sustain Chem Eng 10(15):4994–5004
Wang P-F, Yao H-R, Liu X-Y et al (2018) Na+/vacancy disordering promises high-rate Na-ion batteries. Sci Advances 4(3):eaar6018
Huang Y, Yan Z, Luo W et al (2020) Vitalization of P2–Na2/3Ni1/3Mn2/3O2 at high-voltage cyclability via combined structural modulation for sodium-ion batteries. Energy Storage Mater 29:182–189
Clément RJ, Billaud J, Robert AA et al (2016) Structurally stable Mg-doped P2-Na2/3Mn1−yMgyO2 sodium-ion battery cathodes with high rate performance: insights from electrochemical, NMR and diffraction studies. Energ Environ Sci 9(10):3240–3251
Bianchini M, Wang J, Clément R et al (2018) A first-principles and experimental investigation of nickel solubility into the P2 NaxCoO2 sodium-ion cathode. Adv Energy Mater 8(26):1801446
Guo S, Chen Y, Tong L et al (2022) Biomass hard carbon of high initial coulombic efficiency for sodium-ion batteries: preparation and application. Electrochimica Acta 2022(410):140017
Pei Q, Lu M, Liu Z et al (2022) Zhong, Improving the Na0.67Ni0.33Mn0.67O2 cathode material for high-voltage cyclability via Ti/Cu codoping for sodium-ion batteries. ACS Appl Energy Mater 5(2):1953–1962
Funding
This work was supported by the State Key Program of the National Nature Science of China (Grant No.61835014), the Major Program of the National Natural Science Foundation of China (Grant No.51890865), and the Fundamental Science on Nuclear Wastes and Environmental Safety Laboratory, Southwest University of Science and Technology, Mianyang of China (Grant No.20kfhk01).
Author information
Authors and Affiliations
Contributions
Shihong Guo: conceptualization, investigation, writing – original draft. Huawei Han: conceptualization, investigation, writing – original draft. Yimeng Chen: investigation. Shuai Guo: validation. Ningyun Hong: resources. Jiangtao Fan: data curation. Xiubao Jiang: data curation. Zhen Long: visualization, project administration. Xiaoqing Qiu: validation, writing – review and editing. Mei Tang: project administration. Shifu Xiong: project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
The Supproting Information is available free of charge at Physical parameters, SEM images, Powder XRD patterns of NMO, NMTO-0.10 and NKMTO-0.010 samples, Comparison table of electrochemical properties of different materials, Pseudocapacitive capacities of samples at 0.8 mV s-1 scan rate of NMO. Ratio of the pseudocapacitive and diffusion-controlled contribution at different scan rates of NMO. (DOCX 3470 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, S., Han, H., Guo, S. et al. Improving rate capability and cycling stability of P2-type sodium-ion layered cathode by synergistic effect of K/Ti co-doping strategy. Ionics 29, 2735–2746 (2023). https://doi.org/10.1007/s11581-023-05032-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05032-9